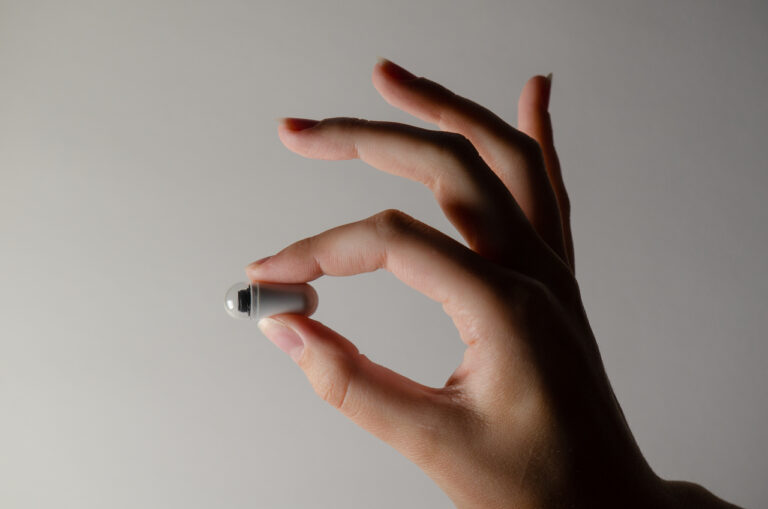
Endoskopowe kapsułki wspierane sztuczną inteligencją mogą uratować życie wielu pacjentów, wykrywając zawczasu groźne schorzenia przewodu pokarmowego. Choć idea tej nieinwazyjnej metody badań narodziła się ponad 20 lat temu, ze względu na szereg ograniczeń wciąż nie jest ogólnodostępna, bo wymaga ogromnych nakładów finansowych i ogranicza się tylko do niektórych odcinków układu pokarmowego. Procesy diagnostyczne w coraz większym stopniu wspierane są jednak przez algorytmy AI, a rewolucji badań endoskopowych przewodzi polski BioCam.
Technologia spółki wróży przełom nie tylko w medycynie ludzkiej, ale i weterynaryjnej. Firma zapowiada komercyjny debiut swojego rozwiązania już na Q4 2024 r.
“Prowadzimy rozmowy o współpracy komercyjnej z liderami Europy Środkowo-Wschodniej w produkcji i dystrybucji leków dla zwierząt. Staramy się jednocześnie o grant w wysokości ok. 20 mln PLN na dopasowanie technologii do rynku weterynaryjnego i komercjalizację trzech różnych kapsułek dla dużych i małych psów, kotów, oraz koni. Równoległy rozwój drugiej ścieżki biznesowej możliwy jest m.in. dzięki mniejszym wymaganiom regulacyjnym na rynku weterynaryjnym. Stąd nie wykluczamy, że przed szerokim wdrożeniem inteligentnej kapsułki dla ludzi, skomercjalizujemy ją wśród czworonogów.”
– podkreśla Maciej Wysocki, prezes medtechowej spółki
Dwutorowe działania BioCamu napędzają prace nad technologią i optymalizacją jej funkcjonowania.
Udoskonalony w obszarach zarówno software, jak i hardware produkt, w styczniu 2024 r. testowany będzie na kolejnych 120 osobach, a w pierwszej połowie przyszłego roku poddany międzynarodowym badaniom klinicznym.
Runda w drodze na NewConnect
O ile samo zaaplikowanie kapsułki jest banalnie proste, o tyle budowa zintegrowanego, inteligentnego systemu to już skomplikowana i czasochłonna praca zespołu naukowców, inżynierów i specjalistów data science. BioCam negocjuje właśnie kolejne kroki finansowania swojej działalności. Spółka domyka rundę inwestycyjną na maksymalnie 3 mln PLN, by przyspieszyć prace nad dopracowaniem technologii do poziomu finalnej kapsułki produkcyjnej. Następnie – bo już nawet latem 2024 r. – BioCam zapowiada swój debiut na rynku
NewConnect i dołączenie do grona wysokotechnologicznych firm na giełdzie
“Patrzymy na historie i dokonania ScopeFluidics czy Bioceltix, które dają inwestorom niebagatelny zwrot z kapitału, a jednocześnie korzystają z finansowania zdobywanego na giełdzie. To też nasza ścieżka rozwoju, z Rynkiem Głównym GPW na horyzoncie. Zapewni nam środki na jeszcze szybszy rozwój umożliwiając inwestycje na znacznie większą skalę. W przypadku medtechów liczy się innowacyjność technologii, możliwość szybkiego skalowania i międzynarodowy potencjał. Na teraz, patrząc na suche fakty, mamy imponującą efektywność finansową. Nasza globalna konkurencja potrzebowała bowiem średnio ok. 100 mln USD, by doprowadzić do podobnego poziomu TRL (gotowości technologicznej) sam hardware, czyli tylko kapsułkę. My przy kilkudziesięciokrotnie mniejszych nakładach mamy lepsze efekty – rozwijamy zintegrowany system zawierający również oprogramowanie AI do analizy obrazów, platformę telemedyczną i mobilną aplikację.” – zaznacza Maciej Wysocki
Do tej pory BioCam pozyskał łącznie ponad 10,5 mln PLN finansowania. Część tej kwoty pochodziła z publicznych dotacji na realizację projektów B&R, a większość od aniołów biznesu i inwestorów VC, w tym funduszu Level2 Ventures. W ciągu ostatniego roku medtech założony w 2019 r. przez Macieja Wysockiego, Roberta Stachurskiego, Jakuba Niemczuka oraz prof. Marka Langnera blisko dwukrotnie powiększył zespół, wzmacniając team techniczny i medyczny pod kątem procesu anotacji. Ostatnio BioCam wziął udział w prestiżowym programie Google for Startups Growth Academy: AI for Health, jako jedna z nielicznych firm z całego regionu EMEA.
Badanie kilkukrotnie tańsze od rynkowej konkurencji
“Istotnym udoskonaleniem technologicznym naszej kapsułki jest m.in. inteligentny sensor obrazu, powodujący jej automatyczne wygaszanie się w przypadku gdy tymczasowo utknie na pewnym odcinku układu pokarmowego. Wydłużyliśmy tym samym czas pracy baterii nawet o kilka godzin. Intensywnie pracujemy ponadto nad technologią Narrow Band Imaging (NBI), umożliwiającą oświetlanie tkanek różnymi długościami światła, dając dodatkową wartość diagnostyczną dzięki wizualizacji zmian patologicznych, niemożliwych do zobaczenia w świetle widzialnym. NBI to standard najbardziej zaawansowanych systemów tradycyjnej endoskopii, ale jeszcze nikt na świecie nie użył jej w endoskopii kapsułkowej.” – komentuje szef BioCam i podkreśla, że prace badawczo-rozwojowe, a także każdy przebadany pacjent przybliża firmę do uzyskania certyfikacji medycznych w Unii Europejskiej i Stanach Zjednoczonych, oraz pierwszego wdrożenia systemu endoskopowego na rynek komercyjny już pod koniec 2024 r. Plany obejmują też starania o refundację – w tej perspektywie oparte na AI badanie zaprojektowane przez BioCam to koszt 1,2–1,5 tys. PLN, czyli kilkukrotnie mniej w porównaniu do konkurencyjnych kapsułek.